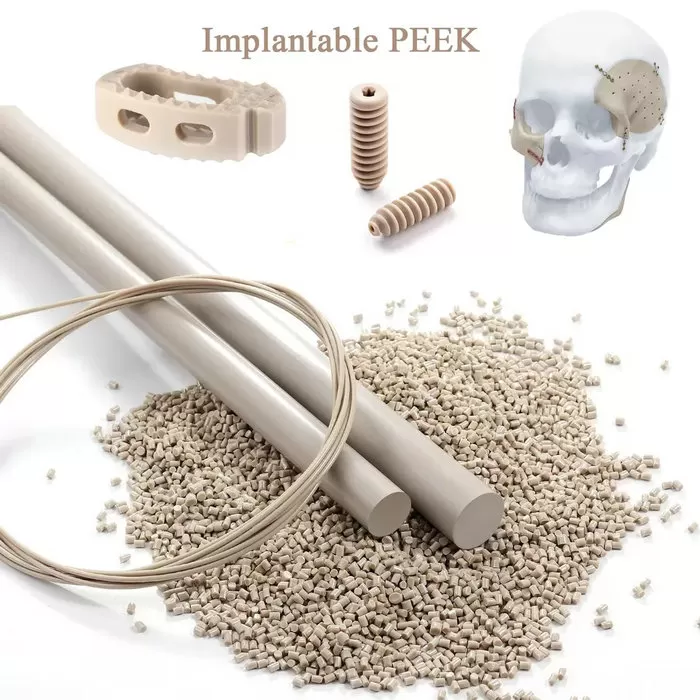


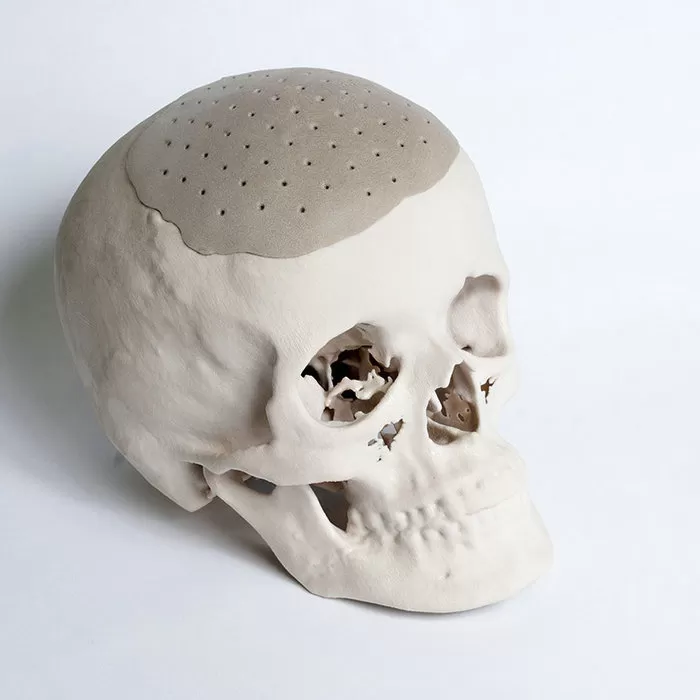
Medical-grade PEEK Materials
ARK-BioPEEK Medical PEEK, or polyetheretherketone, is a high-performance thermoplastic material that is widely used in the medical industry for its exceptional properties. PEEK is known for its biocompatibility, radiolucency, and strength, making it an ideal choice for a variety of medical applications such as spinal implants, dental implants, and orthopedic devices.
Designed to meet the demanding requirements of surgical procedures, our medical grade PEEK exhibit exceptional biocompatibility, allowing for safe and reliable implantation within the human body. The mechanical strength of ARK-BioPEEK ensures robustness and durability, offering the necessary support for surgical implants to withstand various physiological stresses.
With thermal stability as a key characteristic, our medical PEEK materials maintain their structural integrity even under challenging conditions, enabling long-term performance and stability within the implant environment. The materials' resistance to degradation and wear enhances their longevity, providing confidence to healthcare professionals and patients alike.
ARK-BioPEEK prioritize the well-being and safety of patients, which is why our PEEK polymers undergo rigorous testing and validation to guarantee their suitability for surgical implant applications. Our commitment to innovation drives us to continually enhance the performance and properties of our PEEK materials, ensuring they remain at the forefront of surgical implant solutions.
Choose ARK-BioPEEK for your surgical implant needs and experience the benefits of next-generation PEEK materials. Trust in our expertise, reliability, and dedication to delivering high-quality PEEK polymers that redefine the standards of surgical implant applications.
| Properties Information | ||||||
| Property | Reference Standard | Test Method | Unit | Specification | Result | |
| Physical Properties | Glass Transition | ASTM F2026 | ASTM D3418 | °C | 125-165 | 147 |
| Temperature, Tg | ||||||
| Melt Temperature, Tm | ASTM F2026 | ASTM D3418 | °C | 320-360 | 338 | |
| Recrystallization | ASTM F2026 | ASTM D3418 | °C | 260-320 | 289 | |
| Temperature, Tc | ||||||
| Viscosity | ASTM F2026 | ISO 11443 | Pa·s | 400-480 | 437 | |
| Infrared Spectrum | ASTM F2026 | ASTM F1579 | / | See Appendix X1 | See Appendix X2 | |
| Density | ASTM F2026 | ASTM D1505 | kg/m3 | 1280-1320 | 1294 | |
| Chemical Properties | Total Heavy Metals (Ag, As, Bi, Cd, Cu, Hg, Mo, Pb, Sb, and Sn), max | ASTM F2026 | US Pharmacopeia, | ppm | <100 | <10 |
| Test 233 | ||||||
| Mechanical Properties | Tensile Strength at Yield (zero slope), min | ASTM F2026 | ASTM D638, Type IV, 5.08 cm/min | MPa | 90 | 105 |
| Tensile Strength at Break, min | ASTM F2026 | ASTM D638, Type IV, 5.08 cm/min | MPa | 70 | 80 | |
| Elongation at Break, min | ASTM F2026 | ASTM D638, Type IV, 5.08 cm/min | % | 5 | 18 | |
| Flexural Strength, min | ASTM F2026 | ASTM D790 | MPa | 110 | 163 | |
| Flexural Modulus, min | ASTM F2026 | ASTM D790 | GPa | 3 | 4 | |
| Impact Strength, | ASTM F2026 | ISO 180 | kJ/m2 | 4 | 9 | |
| Notched Izod, min | ||||||
| Biological Properties | Genotoxicity | ISO 10993-3 | ISO 10993-3 | / | Negative | Negative |
| Animal Intracutaneous (Intradermal) Reactivity | ISO 10993-10 | ISO 10993-10 | / | ≤1 | 0 | |
| Skin Sensitization | ISO 10993-10 | ISO 10993-10 | / | ≤1 | 0 | |
| Acute Systemic Toxicity | ISO 10993-11 | ISO 10993-11 | / | No Acute Systemic Toxicity | No Acute Systemic Toxicity | |
| Subchronic Systemic Toxicity | ISO 10993-11 | ISO 10993-11 | / | No Subchronic Systemic Toxicity | No Subchronic Systemic Toxicity | |
| local Effects After Implantation | ISO 10993-6 | ISO10993-6 | / | No obvious difference between the test sample and the control sample | No obvious difference between the test sample and the control sample | |
| In Vitro Cytotoxicity | ISO 10993-5 | ISO10993-5 | / | ≤1 | 1 | |
| Evaluation of Haemolytic Properties | ISO 10993-4 | ISO10993-4 | % | <5 | 1 | |
| Material Mediated Pyrogens | ISO 10993-11 | ISO 10993-11 | / | No Pyrogenic Responses | No Pyrogenic Responses | |
| Extractables of The Material | ISO 10993-18 | ISO 10993-18 | μg/g | The contents of Phenyl Sulfone≤300 | The contents of Phenyl Sulfone<0.09 | |