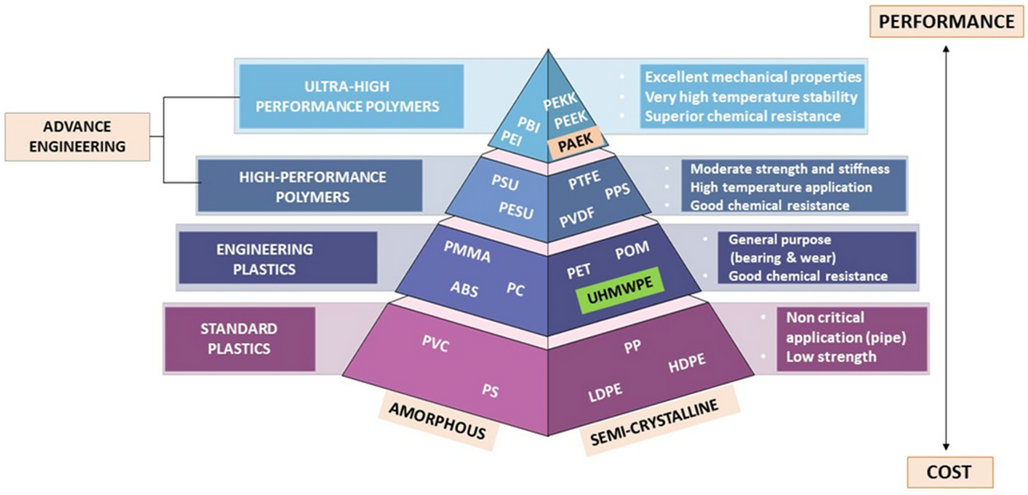
Polyaryletherketone (PAEK) resins, developed in the 20th century, are a class of high-performance engineering plastics renowned for their excellent heat resistance, corrosion resistance, wear resistance, and biocompatibility. They are widely used across defense, aerospace, electronics, automotive, mechanical, oil, nuclear, and medical industries.
PAEK polymers are typically synthesized through A2+B2 nucleophilic substitution reactions using bisphenol and difluoride monomers. The molecular structure significantly influences the properties. Semi-crystalline structures typically consist of regular ether-ketone-benzene chains, while amorphous variants arise from irregular side groups disrupting crystallinity.
PAEK is a linear thermoplastic polymer composed of aromatic rings connected by ether and ketone groups. Its rigid molecular chain and strong intermolecular forces grant excellent thermal resistance and mechanical strength. Ether groups impart toughness—more ethers mean greater flexibility.
PEEK (Polyetheretherketone)
PEK (Polyetherketone)
PEKK (Polyetherketoneketone)
PEEKK (Polyetheretherketoneketone)
PEKEKK (Polyetherketoneetherketoneketone)
Commercialized in the 1980s by ICI, PEEK is a semi-crystalline polymer with Tg = 143°C, Tm = 343°C, and up to 48% crystallinity. Its structure provides excellent heat resistance, mechanical strength, radiation resistance (up to 10⁹ rad), chemical resistance (except concentrated sulfuric acid), dimensional stability, fatigue and wear resistance, and electrical properties. It is extensively applied in aerospace, nuclear energy, electronics, and automotive sectors.
Introduced in 2002 by Victrex (formerly ICI), PEK (PEEK-HT) has a higher Tg of 157°C and Tm of 374°C. It offers better high-temperature strength and wear resistance than PEEK, with slightly higher cost (~10%).
Developed by DuPont in the 1980s, PEKK features a Tg of 165°C and Tm of 381°C. It provides exceptional thermal stability and chemical resistance and is used in high-temperature structural and insulation materials.
Developed by Jilin University, PEEKK builds on PEEK’s properties, with Tg = 162°C and Tm = 367°C. Certain variants reach up to Tg = 192°C, Tm = 428°C, and thermal decomposition temperature of 540°C, making it among the most heat-resistant PAEKs.
Created by Victrex using nucleophilic synthesis, PEKEKK (Tg = 162°C, Tm ≈ 384–387°C) is the third generation of PAEK materials. Reinforced composites show heat deformation resistance up to 386°C and short-term use up to 400°C.
PAEK’s unique molecular structure—aromatic and heterocyclic rings, strong bonding energy, rigid segments—results in:
High Tg and decomposition temperatures
Low flammability
Excellent mechanical properties and modulus
Solvent and chemical resistance
Electrical insulation and radiation resistance
Thermal Resistance: Tg = 143°C, Tm = 343°C. With 30% glass or carbon fiber, it withstands 260°C continuously and 300°C short-term.
Mechanical Properties: Tensile strength up to 212 MPa (30% CF), bending strength up to 335 MPa.
Impact Resistance: Excellent, with notched impact strength >200 kg·cm/cm.
Self-Lubricating & Wear Resistance: Outstanding under various pressures, speeds, and surface roughness. Carbon fiber-reinforced variants rival polyimide.
Chemical Resistance: Insoluble in most solvents; corrosion resistance similar to nickel steel.
Flame Retardancy: UL V-0 (1.45 mm sample without flame retardant).
Hydrolysis Resistance: Maintains performance in hot water and steam (up to 250°C for thousands of hours).
Electrical Insulation: Dielectric loss at 10 Hz = 0.0033; breakdown voltage = 17 kV/mm.
Radiation Resistance: Tolerates >1100 Mrad without insulation loss.
Adhesion and Fatigue Resistance: Strong metal adhesion and excellent fatigue life.
Processability: Suitable for injection molding, extrusion, compression molding, blow molding, melt spinning, rotational molding, and powder coating.
Tg and Tm are closely tied to the ether/ketone ratio. More ketone groups = higher rigidity and heat resistance. However, too many reduce processability. Optimal balance is crucial.
Incorporating biphenyl units alters the chain connection style. For example, comparing PEDEK vs. PEDEKK (same ether/ketone ratio), structural differences affect Tg and Tm. Biphenyl structures next to ketones significantly increase rigidity and thermal performance.
PAEK resins like PEEKK can have increased tensile strength and modulus with higher ketone content. For high-strength applications, fiber reinforcement is recommended.
Crosslinkable PAEK variants (with phenylacetylene groups) allow for tunable thermal performance after crosslinking, increasing Tg further with higher alkyne content.