
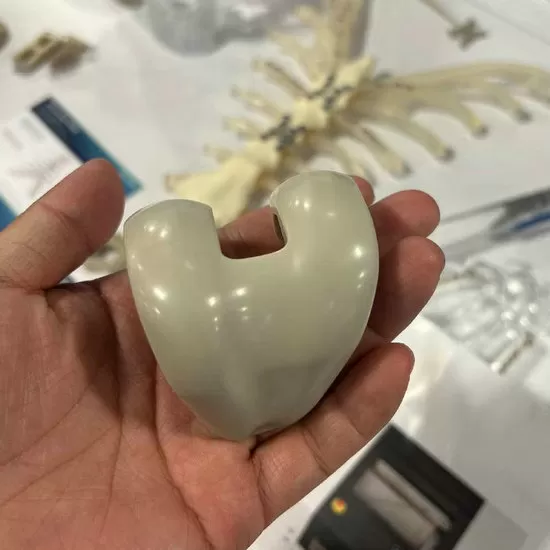



Material: AKSOPEEK
Certificate: FDA, ISO 13485, ISO10993, ASTM F2026
We offer advanced 3D printing services for medical-grade implant parts using AKSOPEEK, a premium PEEK material known for its excellent biocompatibility, mechanical strength, and radiolucency. Ideal for orthopedic, spinal, and reconstructive implants, AKSOPEEK parts provide superior patient comfort and long-term durability.
Revolutionizing Implant Solutions with 3D Printed AKSOPEEK™ Medical Devices
Our cutting-edge service specializes in the design and additive manufacturing of patient-specific medical implant components using certified AKSOPEEK™ – a premium implant-grade polyetheretherketone (PEEK) material. Leveraging industrial 3D printing technology, we overcome traditional manufacturing limitations to produce complex, anatomically precise implants for orthopedic, spinal, craniomaxillofacial (CMF), and dental applications.
Why AKSOPEEK™
Biocompatibility: Meets ISO 10993 standards for long-term implantation
Radiolucency: Enables clear post-op imaging (CT/MRI compatibility)
Bone-like Elasticity: Mimics cortical bone modulus (3–4 GPa) to prevent stress shielding
Chemical Resistance: Withstands aggressive sterilization (autoclave, gamma, EtO)
Osseointegration: Surface-engineered for optimal bone ongrowth
Technical Advantages of 3D Printing
Patient-Specific Design: Implants digitally modeled from patient DICOM data for perfect anatomical conformity
Complex Geometries: Lattice structures for bone ingrowth and fluid channels inaccessible via machining
Rapid Prototyping: Accelerated design-to-implant workflow (3–5 weeks)
Material Efficiency: Near-net-shape production minimizes waste of high-cost PEEK
Applications
Spinal: Interbody cages, laminoplasty plates
Orthopedic: Joint reconstruction, trauma fixation
CMF: Cranial plates, mandibular prosthetics
Dental: Custom abutments, implant guides
Quality Assurance
Manufactured in ISO Class 7 cleanrooms with full traceability from material lot to patient. Compliant with ISO 13485:2016, FDA 21 CFR Part 820, and MDR/IVDR requirements. Every implant undergoes rigorous mechanical validation (ASTM F2026) and surface characterization.
Partner with us for smarter implant solutions – combining the material excellence of AKSOPEEK™ with the design freedom of additive manufacturing to elevate surgical outcomes, reduce OR time, and improve patient quality of life.