_______________________________________________________________________________________________________________________________________________________________________
Have you ever encountered this dilemma?
You’re selecting materials, and the data sheet shows a Tg (glass transition temperature) of 143°C and an HDT (heat deflection temperature) of 160°C. But the customer says the part will be used at 150°C. You hesitate—is this temperature safe? Can PEEK handle it?
Or perhaps during testing, the HDT seems sufficient, but under real-world heat, the part softens and deforms.
It’s confusing—both values seem to describe “heat resistance,” yet the numbers are very different. So, why are both necessary for design?
Let’s break it down and truly understand the difference between Tg and HDT—not just by definition but by material behavior.
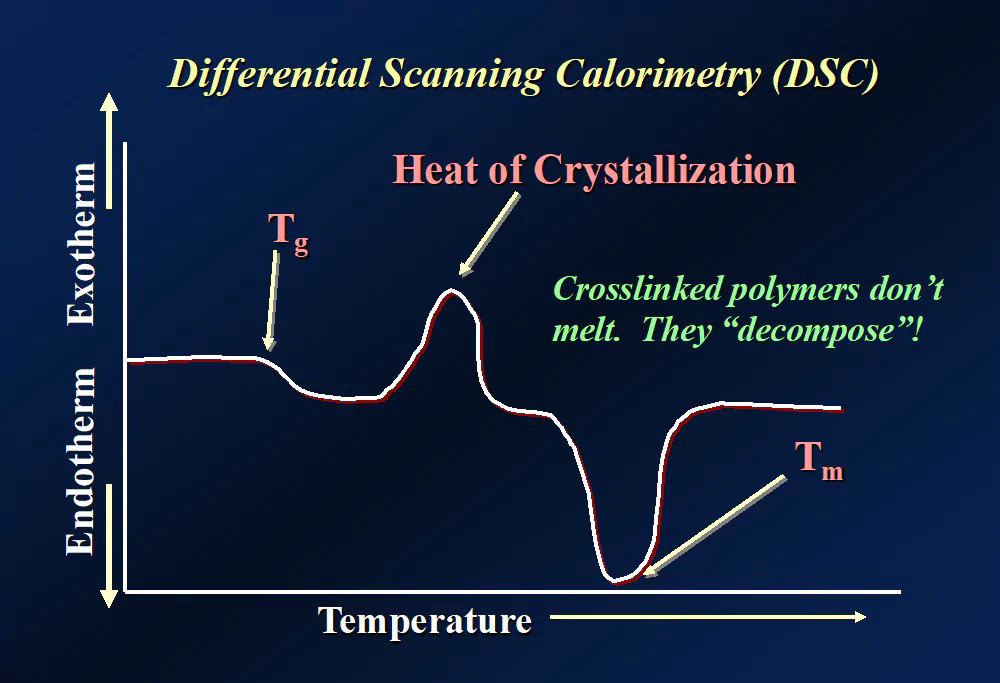
The Tg of PEEK marks the point at which its amorphous chain segments begin to move.
At low temperatures, PEEK’s amorphous regions behave like frozen glass. The molecules only vibrate locally—they can’t rotate or relax. But once the temperature reaches Tg, the molecular motion is activated. The material enters the rubbery state, becomes more flexible, and its modulus significantly drops.
At Tg, important property changes may occur:
Increased flexibility
Changes in electrical or barrier properties
Enhanced gas permeability
But Tg doesn’t tell you if the material can still bear load. It only signals that the molecules are moving.
HDT is a macro-level measure of mechanical performance under heat and load.
It tells you: under simultaneous heat and stress (usually 1.8 MPa), when does the material start to deform significantly? HDT marks the beginning of that structural collapse.
HDT is affected by more than just chain movement:
Crystalline regions (which act like structural reinforcements)
Glass fiber or mineral fillers (which boost rigidity)
Crosslinking (which locks the structure in place)
Even if molecular chains are already moving post-Tg, PEEK’s semi-crystalline regions can still resist deformation. For example, PEEK has a Tg of 143°C but can be used continuously up to 260°C, thanks to its crystalline structure.
For amorphous plastics, when Tg is reached, performance often collapses. But for semi-crystalline PEEK, the crystal zones remain strong even after Tg is crossed.
| Dimension | Tg (Glass Transition Temperature) | HDT (Heat Deflection Temperature) |
| What it describes | When molecular chains become active | When the whole structure starts collapsing |
| Fundamental meaning | Microscopic physical state shift | Macroscopic loss of mechanical rigidity |
| Test conditions | No load, purely thermal response | Constant load applied, temperature increasing |
| Key influencing factors | Chain flexibility, free volume | Fillers, crystallinity, crosslinks, modulus |
| Application | Identifying phase state, material behavior | Determining if it can hold shape under heat |
In short:
Tg tells you if the molecules are moving.
HDT tells you if the structure is collapsing.
For PEEK, Tg is 143°C, but HDT can go well over 160°C. Why?
Tg is mostly determined by the onset of chain mobility.
HDT heavily depends on structural rigidity—reinforced by crystallinity, fillers, and other macro-level features.
Here’s what you need to know:
Fillers and crystallinity have little effect on Tg, but greatly increase HDT
Semi-crystalline materials (like PEEK) may have low Tg but high HDT
Amorphous materials (like PC, PMMA) tend to have Tg and HDT close together
Think of Tg as when you start sweating.
Think of HDT as when your legs give out and you collapse.
Can the part hold shape under heat? Go by HDT.
Example: brackets, fixtures, carriers, fasteners.
Will the part become soft or flexible? Focus on Tg.
Example: thermal sealing films, coatings, flexible circuits.
Molding temperature must be above Tg to allow flow
Secondary processes (e.g., post-machining) must be below HDT to prevent collapse
Don’t fall for vague “heat resistance” claims. PEEK’s Tg, HDT, and even melting point (Tm) each describe different aspects of thermal behavior.
Tg: when the molecular chains loosen
HDT: when the part loses its structural strength
Tm: when the material fully melts
As engineers, it’s critical to build a thermal response mindset—not just chase a single number.